1-Benzylisoquinoline
From DrugPedia: A Wikipedia for Drug discovery
m (1 revision) |
(→External Links) |
||
| (3 intermediate revisions not shown.) | |||
| Line 6: | Line 6: | ||
{{Drugbox | {{Drugbox | ||
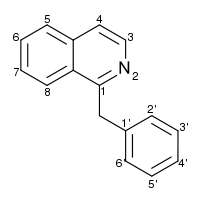
| IUPAC_name =1-(phenylmethyl)isoquinoline | | IUPAC_name =1-(phenylmethyl)isoquinoline | ||
| + | |image=Benzylisoquinoline structure.png | ||
| + | | width=250 | ||
| + | | CAS_number = 6907-59-1 | ||
| PubChem = 23345 | | PubChem = 23345 | ||
| DrugBank = | | DrugBank = | ||
| - | | ChemSpiderID = | + | | ChemSpiderID = 21830 |
| chemical_formula =C<sub>1</sub><sub>6</sub>H<sub>1</sub><sub>3</sub>N | | chemical_formula =C<sub>1</sub><sub>6</sub>H<sub>1</sub><sub>3</sub>N | ||
| molecular_weight = 219.28112 | | molecular_weight = 219.28112 | ||
| smiles = N/A | | smiles = N/A | ||
| - | | synonyms = | + | | synonyms = 1-(phenylmethyl)isoquinoline |
| density = | | density = | ||
| melting_point = | | melting_point = | ||
| Line 24: | Line 27: | ||
==Description== | ==Description== | ||
| + | '''1-Benzyl[[isoquinoline]]''' is the structural backbone of many [[alkaloid]]s with a wide variety of structures, including [[papaverine]], [[noscapine]], [[codeine]], [[morphine]], [[apomorphine]], [[berberine]], [[protopine]] and [[tubocurarine]]. | ||
| + | ==Biosynthesis== | ||
| + | Plants producing benzylisoquinoline alkaloids have a common biosynthetic pathway, making use of two units of <small>L</small>-[[tyrosine]]. One tyrosine molecule is metabolised to [[dopamine]] which constitutes the isoquinoline part, while the benzylic part is mostly formed from [[tyramine]], itself the decarboxylation product of tyrosine. | ||
| + | |||
| + | Many benzylisoquinolines have a [[methyl]]ated nitrogen atom as well as functional groups containing oxygen ([[Hydroxyl|-OH]], [[Methoxy|-OCH<sub>3</sub>]], -OCH<sub>2</sub>O-) in positions 6, 7, 3' and 4'. The latter come from the precursors mentioned above, namely tyrosine, dopamine and their derivatives. | ||
==General Properties== | ==General Properties== | ||
| Line 60: | Line 68: | ||
[http://crdd.osdd.net/raghava/biadb/detail.php?id=1319 Link to BIAdb Database] | [http://crdd.osdd.net/raghava/biadb/detail.php?id=1319 Link to BIAdb Database] | ||
| + | [[Category:BIAdb_old]] | ||
| + | |||
| + | ==References== | ||
| + | *[http://epub.ub.uni-muenchen.de/3630/1/010.pdf Benzylisoquinoline biosynthesis by cultivated plant cells and isolated enzymes] | ||
| + | * Organic Syntheses, Coll. Vol. 6, p. 115, 1988 | ||
| + | [[Category:Alkaloids]] | ||
Current revision
| |
| 1-Benzylisoquinoline
| |
| Systematic (IUPAC) name | |
| 1-(phenylmethyl)isoquinoline | |
| Identifiers | |
| CAS number | |
| ATC code | ? |
| PubChem | |
| ChemSpider | |
| Chemical data | |
| Formula | C16H13N |
| Mol. mass | 219.28112 |
| SMILES | & |
| Synonyms | 1-(phenylmethyl)isoquinoline |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Contents |
[edit] Description
1-Benzylisoquinoline is the structural backbone of many alkaloids with a wide variety of structures, including papaverine, noscapine, codeine, morphine, apomorphine, berberine, protopine and tubocurarine.
[edit] Biosynthesis
Plants producing benzylisoquinoline alkaloids have a common biosynthetic pathway, making use of two units of L-tyrosine. One tyrosine molecule is metabolised to dopamine which constitutes the isoquinoline part, while the benzylic part is mostly formed from tyramine, itself the decarboxylation product of tyrosine.
Many benzylisoquinolines have a methylated nitrogen atom as well as functional groups containing oxygen (-OH, -OCH3, -OCH2O-) in positions 6, 7, 3' and 4'. The latter come from the precursors mentioned above, namely tyrosine, dopamine and their derivatives.
[edit] General Properties
*Molecular Weight
219.28112
*Molecular Formula
C16H13N
*IUPAC NAME
1-(phenylmethyl)isoquinoline
*Canonical Smiles
C1=CC=C(C=C1)CC2=NC=CC3=CC=CC=C32
*Isomeric Smiles
N/A
*XLogP
3.9
*Topological Polar Surface Area
12.9
[edit] External Links
[edit] References
- Benzylisoquinoline biosynthesis by cultivated plant cells and isolated enzymes
- Organic Syntheses, Coll. Vol. 6, p. 115, 1988

