Mtb cell wall
From DrugPedia: A Wikipedia for Drug discovery
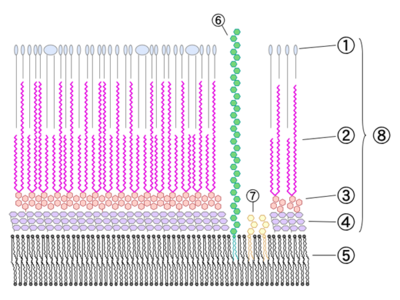
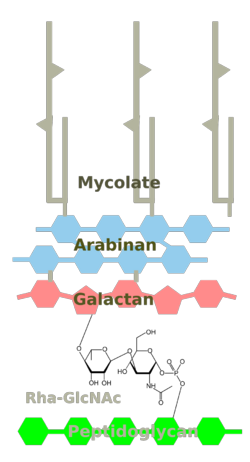
The cell wall consists of several layers (from outer, see Fig. 1):
Contents |
1. Phthiocerol Dimycocerosate (DIM/DIP) layer
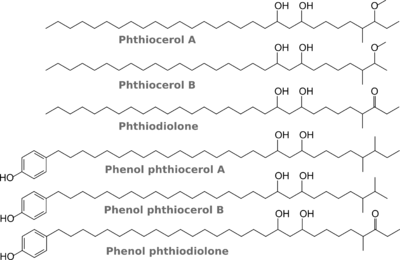
DIM are esters of phthio compounds (Fig. 2) -- which are diols -- with methyl-branched fatty acids. The phenylphthio compounds can be glycosylated too. In all other species that have similar compounds, the chain length is shorter, except in M. bovis.
Indeed, unlike DIM, which are produced by all strains of M. tuberculosis examined to date, only a very small proportion of M. tuberculosis, in particular the Canetti and related strains of M. tuberculosis, elaborate phenolphthiocerol dimycocerosates, or their glycosylated derivatives.(Daffé et al 1987)
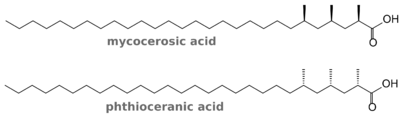
As to the estered acids, it's 2,4,6-trimethyl- and 2,4,6,8-tetramethyloctacosanoic acid (Fig. 3). Only M. marinum and ulcerans feature the L-form which is called phthioceranic acid; the name of the D-form is mycocerosic acid.
So, to put it all together for Mtb, the outermost layer of the Mtb cell wall consists of dimycocerosates of phthio compounds, where phthiocerol A/B and phthiodiolone have 21 and 23 C-atoms left of the hydroxymethyl group; the phenylphthio compounds have 14-18 C-atoms between the benzene ring and the hydroxymethyl group; and finally, the mycocerosic acid is both the trimethyl and tetramethyl sort.
We cannot find, at the moment, any information about stereoisomerism at the hydroxy- and methyl-/ethyl groups of the phthio compounds. It seems no one has bothered about that until now.
2/3. Arabinogalactan dimycolate layer
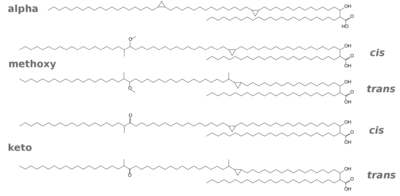
Mycolic acids (Fig. 5) are the longest fatty acids known from nature. They feature also cyclopropane residues in the chain which is unusual (see Grogan/Cronan 1997 for an early review of such compounds). Shown here are the five types of mycolic acids found in Mtb. The mycolic acids are bound as esters to arabinogalactan, so the layer actually consists of arabinogalactan mycolate. There is also a free glycolipid called trehalose dimycolate (TDM, cord factor), which accumulates in a cord-like fashion on the surface of the cells.
Three distinct structural classes of mycolic acids are found in M. tuberculosis, and they are α-, methoxy- and keto-mycolic acids, as shown in Fig. 6. The α-mycolic acid is the most abundant form (>70%), whereas methoxy- and keto-mycolic acids are the minor components (10 to 15%). For an excellent review of the biosynthetic pathways see Takayama et al.(2005).
The H37R strains may have been laboratory-selected for lower trans-cyclopropylmycolate content (versus cis) with a higher permeability cell wall and faster growth rate (Yuan 1997).
4. Peptidoglycan
5. Plasma membrane (?)
6. Lipoarabinomannan
Lipoarabinomannan (LAM) as well as its related precursors, lipomannan (LM) and phosphatidyl-myo-inositol mannosides (PIMs), are found interspersed in the mycobacterial cell wall. See the excellent review by Briken et al.(2004).
7. Phosphatidylinositol Mannoside
References
- Briken V, Porcelli SA, Besra GS, and Kremer L (Jul 2004). "Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response". Mol Microbiol, 53(2):391–403. PMID 15228522
- Daffée M and Lanéelle MA (July 1988). "Distribution of phthiocerol diester, phenolic mycosides and related compounds in mycobacteria". J Gen Microbiol. 134 (7): 2049-55. PMID 3149973
- Daffé, M., Lacave, C., Lanéelle, M.-A., and Lanéelle, G. (1987). Structure of the major triglycosyl phenol-phthiocerol of Mycobacterium tuberculosis (strain Canetti). Eur. J. Biochem. 167, 155–160. PMID 3113946
- Glickman MS, Cahill SM, Jacobs WR (January 2001). "The Mycobacterium tuberculosis cmaA2 gene encodes a mycolic acid trans-cyclopropane synthetase". J. Biol. Chem. 276 (3): 2228–33. DOI:10.1074/jbc.C000652200. PMID 11092877
- Grogan DW and Cronan JE Jr (Dec 1997). "Cyclopropane ring formation in membrane lipids of bacteria." Microbiol Mol Biol Rev, 61(4):429–41, PMID 9409147
- Takayama K, Wang C, and Besra GS (January 2005). Pathway to Synthesis and Processing of Mycolic Acids in Mycobacterium tuberculosis. Clin Microbiol Rev. 18(1): 81–101. DOI: 10.1128/CMR.18.1.81-101.2005. PMCID: PMC544180. PMID 15653820
- Yuan Y, Crane DC, Musser JM, Sreevatsan S, and Barry CE 3rd (Apr 1997). "Mmas-1, the branch point between cis- and trans-cyclopropane-containing oxygenated mycolates in mycobacterium tuberculosis." J Biol Chem, 272(15):10041–9. PMID 9092547