17-Hydroxypregnenolone
From DrugPedia: A Wikipedia for Drug discovery
| Line 61: | Line 61: | ||
CC(=O)[C@]1(CCC2[C@@]1(CC[C@H]3[C@H]2CC=C4[C@@]3(CC[C@@H](C4)O)C)C)O | CC(=O)[C@]1(CCC2[C@@]1(CC[C@H]3[C@H]2CC=C4[C@@]3(CC[C@@H](C4)O)C)C)O | ||
==Prohormone== | ==Prohormone== | ||
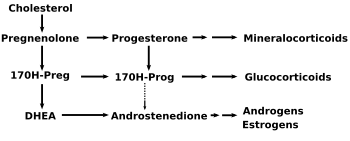
| - | [[Image:DHEA1. | + | [[Image:DHEA1.png|thumb|right|350px|17 OH–pregnenolone is converted from pregnenolone and gives rise to DHEA (below) and to 17 OH-progesterone (to its right)]] |
17-OH-pregnenolone is considered a [[prohormone]] in the formation of [[dehydroepiandrosterone]] (DHEA), itself a prohomone of the [[sex steroid]]s. | 17-OH-pregnenolone is considered a [[prohormone]] in the formation of [[dehydroepiandrosterone]] (DHEA), itself a prohomone of the [[sex steroid]]s. | ||
Current revision
|
|
| 17-Hydroxypregnenolone
| |
| Systematic (IUPAC) name | |
| 1-[(3S,8R,9S,10R,13S,17R)-3,17-dihydroxy-10,13-dimethyl-1,2,3,4,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-yl]ethanone | |
| Identifiers | |
| CAS number | |
| ATC code | ? |
| PubChem | |
| Chemical data | |
| Formula | ? |
| Mol. mass | 332.48 g/mol |
| Physical data | |
| Melt. point | 268 °C |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | AdrenalGonads |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Contents |
[edit] Description
17-Hydroxypregnenolone (also 17-OH-pregnenolone and 17α-hydroxypregnenolone), is a C21 steroid that is obtained by hydroxylation of pregnenolone at the C17α position. This step is performed by the mitochondrial cytochrome P450 enzyme 17α-hydroxylase (CYP17A1) that is present in the adrenal and gonads. Peak levels are reached in humans at the end of puberty and then decline.<ref>Hill M, Lukac D, Lapcik O, Sulcova J, Hampl R, Pouzar V, Starka L. Age relationships and sex differences in serum levels of pregnenolone and 17-hydroxypregnenolone in healthy subjects. Clin Chem Lab Med. 1999 Apr;37(4):439-47. PMID 10369116</ref> High levels are also achieved during pregnancy.
[edit] General Properties
*Molecular Weight
332.48
*Molecular Formula
C21H32O3
*IUPAC NAME
1-[(3S,8R,9S,10R,13S,17R)-3,17-dihydroxy-10,13-dimethyl-1,2,3,4,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-yl]ethanone
*Canonical Smiles
CC(=O)C1(CCC2C1(CCC3C2CC=C4C3(CCC(C4)O)C)C)O
*Isomeric Smiles
CC(=O)[C@]1(CCC2[C@@]1(CC[C@H]3[C@H]2CC=C4[C@@]3(CC[C@@H](C4)O)C)C)O
[edit] Prohormone
17-OH-pregnenolone is considered a prohormone in the formation of dehydroepiandrosterone (DHEA), itself a prohomone of the sex steroids.
This conversion is mediated by the enzyme 17,20 lyase . As such 17-OH-pregenolone represents an intermediary in the delta-5-pathway that leads from pregnenolone to DHEA. 17-hydroxypregneolone is also converted to 17-hydroxyprogesterone, a prohomone for glucocorticosteroids and androstenedione through the activity of 3-hydroxysteroid dehydrogenase.
[edit] Neurohormone
There is some evidence that 17-OH-pregnenolone may have activity as a neurohormone.<ref>Matsunaga M, Ukena K, Baulieu EE, Tsutsui K 7alpha-Hydroxypregnenolone acts as a neuronal activator to stimulate locomotor activity of breeding newts by means of the dopaminergic system. Proc Natl Acad Sci USA 2004 Dec 7;101(49):17282-7. PMID 15569930</ref>
[edit] Clinical use
Measurements of 17-OH-pregnenolone are useful in the diagnosis of certain forms of congenital adrenal hyperplasia.<ref>Riepe FG, Mahler P, Sippell, Partsch CJ. Longitudinal Study of Plasma Pregnenolone and 17-Hydroxypregnenolone in Full-Term and Preterm Neonates at Birth and during the Early Neonatal Period. The Journal of Clinical Endocrinology & Metabolism (2002) 87: 4301-4306
[1]</ref>
In patients with congenital adrenal hyperplasia due to 3 beta-hydroxysteroid dehydrogenase deficiency 17-OH-pregnenolone is increased, while in patients with congenital adrenal hyperplasia due to 17 alpha-hydroxylase deficiency levels are low to absent. IDS