Protein Refolding
From DrugPedia: A Wikipedia for Drug discovery
| (One intermediate revision not shown.) | |||
| Line 1: | Line 1: | ||
| - | Recombinant DNA technology allows the expression of valuable heterologous proteins at high expression rates. Particularly in Escherichia coli (E. coli) overexpression of proteins often leads to aggregation and deposition in dense, insoluble particles within the host cell, so-called inclusion bodies (IB). Formation of inclusion bodies is heavily protein dependent, charge distribution and turn forming residues have a strong impact (1), also presence of cysteines may enforce tendency of aggregate formation (2)but it may also be influenced by altering cell cultivation conditions (3). | + | Recombinant DNA technology allows the expression of valuable heterologous proteins at high expression rates. Particularly in Escherichia coli (E. coli) overexpression of proteins often leads to aggregation and deposition in dense, insoluble particles within the host cell, so-called inclusion bodies (IB). Formation of inclusion bodies is heavily protein dependent, charge distribution and turn forming residues have a strong impact (1), also presence of cysteines may enforce tendency of aggregate formation (2)but it may also be influenced by altering cell cultivation conditions (3). Inclusion bodies are dense aggregates of misfolded polypeptide. They are formed intracellularly because of the aggregating characteristics of the protein or the |
| - | + | inability of the cellular processes to ensure that the expressed polypeptide is soluble and folded correctly.[[Image:inclusion bodies.jpg|thumbnail|100px|]] | |
| - | + | ||
| - | [[Image:inclusion bodies.jpg|thumbnail|100px|]] | + | |
Current revision
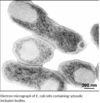
Recombinant DNA technology allows the expression of valuable heterologous proteins at high expression rates. Particularly in Escherichia coli (E. coli) overexpression of proteins often leads to aggregation and deposition in dense, insoluble particles within the host cell, so-called inclusion bodies (IB). Formation of inclusion bodies is heavily protein dependent, charge distribution and turn forming residues have a strong impact (1), also presence of cysteines may enforce tendency of aggregate formation (2)but it may also be influenced by altering cell cultivation conditions (3). Inclusion bodies are dense aggregates of misfolded polypeptide. They are formed intracellularly because of the aggregating characteristics of the protein or the
inability of the cellular processes to ensure that the expressed polypeptide is soluble and folded correctly.
[edit] References
1. Wilkinson, D.L., Harrison, R.G., 1991. Predicting the solubility of recombinant proteins in Escherichia coli. Bio/Technology 9, 443–448.
2. Rinas, U., Tsai, L.B., Lyons, D., Fox, G.M., Stearns, G., Fieschko, J., Fenton, D., Bailey, J.E., 1992. Cysteine to serine substitutions in basic fibroblast growth factor: effect on inclusion body formation and proteolytic susceptibility during in vitro refolding. Bio/Technology 10, 435–440.
3. Panda, A.K., Khan, R.H., Rao, K., Totey, S.M., 1999. Kinetics of inclusion body production in batch and high cell density fed-batch culture of Escherichia coli expressing ovine growth hormone. J. Biotechnol. 75, 161–172.