Swine flu pandemic
From DrugPedia: A Wikipedia for Drug discovery
(→H1N1 Flu (Swine Flu)) |
|||
| (9 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| + | =[http://crdd.osdd.net/drugpedia/index.php/Bioinformatics_Resource_for_Swine_Flu_:_Suggestions_invited Suggestions Invited]= | ||
| + | |||
== H1N1 Flu (Swine Flu)== | == H1N1 Flu (Swine Flu)== | ||
Also called: Swine flu | Also called: Swine flu | ||
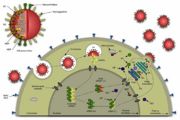
| + | [[Image:influenza_life_cycle_overview.jpg|thumb|Life cycle of influenza virus]] | ||
Swine flu is an infection caused by a virus. It's named for a virus that pigs can get. People do not normally get swine flu, but human infections can and do happen. The virus is contagious and can spread from human to human. Symptoms of swine flu in people are similar to the symptoms of regular human flu and include fever, cough, sore throat, body aches, headache, chills and fatigue. | Swine flu is an infection caused by a virus. It's named for a virus that pigs can get. People do not normally get swine flu, but human infections can and do happen. The virus is contagious and can spread from human to human. Symptoms of swine flu in people are similar to the symptoms of regular human flu and include fever, cough, sore throat, body aches, headache, chills and fatigue. | ||
| Line 81: | Line 84: | ||
==Benefits of Antiviral Drugs== | ==Benefits of Antiviral Drugs== | ||
Photo of man covering face with tissueTreatment: If you get sick, antiviral drugs can make your illness milder and make you feel better faster. They may also prevent serious influenza complications. Influenza antiviral drugs work best when started soon after illness onset (within two [2] days), but treatment with antiviral drugs should still be considered after 48 hours of symptom onset, particularly for hospitalized patients or people at high risk for influenza-related complications. | Photo of man covering face with tissueTreatment: If you get sick, antiviral drugs can make your illness milder and make you feel better faster. They may also prevent serious influenza complications. Influenza antiviral drugs work best when started soon after illness onset (within two [2] days), but treatment with antiviral drugs should still be considered after 48 hours of symptom onset, particularly for hospitalized patients or people at high risk for influenza-related complications. | ||
| + | |||
| + | ==Frequently Asked question about Antiviral Drug== | ||
| + | 1. For what purposes can antiviral drugs be used against influenza A(H1N1)? | ||
| + | <br> | ||
| + | Ans.So far most people who have contracted the new A (H1N1) virus have experienced influenza-like symptoms (such as sore throat, cough, runny nose, fever, malaise, headache, joint/muscle pain) and recovered without antiviral treatment. | ||
| + | <br> | ||
| + | Antiviral drugs may reduce the symptoms and duration of illness, just as they do for seasonal influenza. They also may contribute to preventing severe disease and death. Influenza A (H1N1) is a new virus and only a small number of people with the infection have been treated for it with antiviral drugs. WHO is in touch with public health authorities and clinicians in affected countries and is gathering information about how effective the drugs are. | ||
| + | <br> | ||
| + | 2.To which antiviral drugs does this influenza virus respond? | ||
| + | <br> | ||
| + | Ans.There are two classes of antiviral drugs for influenza: inhibitors of neuraminidase such as oseltamivir and zanamivir; and adamantanes, such as amantadine and rimantadine. Tests on viruses obtained from patients in Mexico and the United States have indicated that current new H1N1 viruses are sensitive to neuraminidase inhibitors, but that the viruses are resistant to the other class, the adamantanes. | ||
| + | <br> | ||
| + | 3.Could the virus become resistant to oseltamivir and zanamivir? | ||
| + | <br> | ||
| + | Ans.Resistance can develop to antiviral drugs used for influenza. Therefore, WHO and its partners are monitoring antiviral drug resistance. | ||
| + | <br> | ||
| + | 4.Under what circumstances should antiviral drugs be administered? | ||
| + | <br> | ||
| + | Antiviral drugs are to be used according to national pandemic influenza preparedness plans. Public health authorities in some countries have decided to treat patients likely to have this disease as a part of public health measures. | ||
| + | <br> | ||
| + | 5.Where antiviral drugs are available for treatment, clinicians should make decisions based on assessment of the individual patient's risk. Risks versus benefits should also be evaluated on a case by case basis. | ||
| + | Should I take an antiviral now just in case I catch the new virus? | ||
| + | <br> | ||
| + | Ans.No. You should only take an antiviral, such as oseltamivir or zanamivir, if your health care provider advises you to do so. Individuals should not buy medicines to prevent or fight this new influenza without a prescription, and they should exercise caution in buying antivirals over the internet. | ||
| + | <br> | ||
| + | 6.What is WHO doing about getting antiviral drugs to countries as preparation for a pandemic? | ||
| + | <br> | ||
| + | Ans.WHO’s first priority is to provide an emergency stock of antiviral drugs to countries that have no or insufficient stock of the drugs and lack the capacity to procure these drugs themselves. | ||
| + | WHO is also working with Member States, donors and other groups that have stockpiles and are willing to share these with WHO for distribution to countries in need. | ||
| + | <br> | ||
| + | 7.Which drug will be provided, and how much of it does WHO have available? | ||
| + | <br> | ||
| + | Ans.WHO had a global stockpile of approximately 5 million adult treatment courses of oseltamivir. Part of this stockpile has already been distributed through the WHO Regional Offices, which are handling allocation and distribution. WHO is currently distributing the remaining 3 million adult treatment courses of this stockpile to developing countries in need. | ||
| + | <br> | ||
| + | WHO continues to assess needs and to work with manufacturers to secure more donations of antivirals. More antiviral drugs will be distributed once these donations are received. | ||
| + | <br> | ||
| + | 8.Which countries will receive the drug, and how will they be selected? | ||
| + | <br> | ||
| + | Ans.WHO has arranged the first deployment of antiviral drugs from the WHO stockpile to 72 countries. Priority was given to vulnerable countries, taking into consideration national manufacturing and procurement capacity. As necessary, other countries will be supported through regional office stockpiles. | ||
| + | <br> | ||
| + | 9.What if the initial emergency deployment turns out to be inadequate? | ||
| + | <br> | ||
| + | Ans.WHO is in discussion with manufacturers regarding the potential need for scaling up production. It is WHO’s understanding that manufacturers have plans for producing large numbers of treatments quickly. | ||
| + | |||
| + | WHO will work on behalf of its Member States to secure further antivirals as needed, either through donations or purchase at the lowest possible price. | ||
| + | ==Vaccine== | ||
| + | Swine Flu Vaccine | ||
| + | It is estimated that the swine flu vaccine won't be ready until sometime around September to November 2009. In addition to the time required to actually make a new vaccine, the likely need to make seasonal flu vaccine for next year may delay things a little. | ||
| + | |||
| + | Can the swine flu vaccine be combined with the seasonal flu vaccine? No, as vaccine companies will be done making seasonal flu vaccine before they can likely even get started on the swine flu vaccine. | ||
| + | |||
| + | Once the swine flu is ready, who will get it? | ||
| + | |||
| + | CDC’s Advisory Committee on Immunization Practices (ACIP) recommends that swine flu vaccine should first go to: | ||
| + | |||
| + | * Pregnant women | ||
| + | * Household contacts and caregivers for children younger than 6 months of age | ||
| + | * Healthcare and emergency medical services personnel | ||
| + | * All children and young adults from 6 months through 24 years of age, and | ||
| + | * Persons aged 25 through 64 years who have health conditions associated with higher risk of medical complications from influenza | ||
| + | |||
| + | That will take up about 159 million doses of swine flu vaccine. If supplies of swine flu vaccine are limited, priority should go to: | ||
| + | |||
| + | * Pregnant women | ||
| + | * Household contacts and caregivers for children younger than 6 months of age | ||
| + | * Healthcare and emergency medical services personnel | ||
| + | * Children 6 months through 4 years of age, and | ||
| + | * Children 5 through 18 years of age who have chronic medical conditions | ||
| + | Next, as swine flu vaccine improves to the point that all priority groups have gotten vaccinated, everyone from the ages of 25 through 64 years will get vaccinated too. Lastly, people 65 or older, who have the least risk from the swine flu will be offered the swine flu vaccine. | ||
| + | |||
| + | Swine Flu Vaccine Trials | ||
| + | |||
| + | Novartis has started human testing of H1N1 swine flu vaccine candidates while Sanofi-Aventis, the world leader in flu shots, will commence within days, company officials said on Tuesday. | ||
| + | |||
| + | The National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health, will soon start swine flu clinical trials to make sure the new swine flu vaccines are safe and effective. They are to be conducted at eight university research hospitals and medical organizations across the United States, including Baylor College of Medicine in Houston, Children's Hospital Medical Center in Cincinnati, and Emory University in Atlanta. | ||
| + | |||
| + | The first clinical trials will test whether one or two doses are needed and will test both 15mcg and 30mcg doses of vaccine. Although the trials will start in adults, if the vaccines are safe, they will also be tested in children. | ||
| + | 1976 Swine Flu Vaccine | ||
| + | |||
| + | Although it is true that we don't currently have a swine flu vaccine, there once was a swine flu vaccine that was made to target the swine flu H1N1 strain that was found at Fort Dix, New Jersey. Because of fears that this swine flu strain was similar to the flu strain that caused the 1918 Spanish Flu pandemic, a vaccination program immunized more than 40 million people in the United States between October 1976 to December 1976. | ||
| + | |||
| + | The immunization program was stopped early because the swine flu pandemic didn't occur, and the swine flu vaccine was thought to cause many side effects, including Guillain-Barre syndrome. | ||
| + | |||
| + | How many cases of Guillain-Barre syndrome were there? About 40, or 1 per million people vaccinated. Even that was considered too high though in light of the fact that there didn't seem to be any cases of swine flu that season. | ||
| + | |||
| + | ==Some PDB Structure of H1N1== | ||
| + | 1RU7 HA Hemagglutinin H1N1 1934 human h1 hemagglutinin AAA43194.1 | ||
| + | 1RVX HA Hemagglutinin H1N1 1934 h1 hemagglutinin in complex with lsta AAA43194.1 | ||
| + | 1RVZ HA Hemagglutinin H1N1 1934 h1 hemagglutinin in complex with lstc AAA43194.1 | ||
| + | 1AA7 M1 Matrix protein 1 H1N1 influenza virus matrix protein crystal structure at ph 4.0 CAA24282.1 | ||
| + | 1HHI M1 Matrix protein 1 H1N1 the antigenic identity of peptide(slash)mhc complexes: a comparison of the conformation of five peptides presented by hla-a2 AAM75161.1 | ||
| + | 1EA3 M1 Matrix protein 1 H1N1 influenza virus m1 protein AAM75161.1 | ||
| + | 2GX9 NS1 Non-structural protein 1 H1N1 x-ray strucutre of influenza virus ns1 effector domain AAA43536.1 | ||
| + | 1PD3 NS2 Non-structural protein 2 H1N1 influenza a nep m1-binding domain | ||
| + | 3BUY PB1-F2 H1N1 mhc-i in complex with peptide ABO21707.1 | ||
| + | 2HN8 PB1-F2 H1N1 structural characterization and oligomerization of pb1-f2, a pro-apoptotic influenza a virus protein ABO21707.1 | ||
| + | 2H95 M2 Matrix protein2 H1N1 structure of the amantadine-blocked influenza a m2 proton channel trans-membrane domain by solid-state nmr spectroscopy ABD95352.1 | ||
| + | 1NYJ M2 Matrix protein2 H1N1 the closed state structure of m2 protein h+ channel by solid state nmr spectroscopy ABF21315.1 | ||
| + | 2IQH NP Nucleoprotein A/WSN/1933 TS61 H1N1 influenza a virus nucleoprotein np at 3.2a resolution ABF47959.1 | ||
| + | |||
| + | Non structural protein NP1 may the the important drug target as concerned from it function. | ||
| + | function | ||
| + | Inhibits post-transcriptional processing of cellular pre-mRNA, by binding and inhibiting two cellular proteins that are required for the 3'-end processing of cellular pre-mRNAs: the 30 kDa cleavage and polyadenylation specificity factor (CPSF4) and the poly(A)-binding protein 2 (PABPN1). This results in the accumulation of unprocessed 3' end pre-mRNAs which can't be exported from the nucleus. Cellular protein synthesis is thereby shut off very early after virus infection. This protein synthesis shut off is presumably necessary for the virus to escape interferon synthesis after activation of the IRF3 pathway, and therefore prevents establishment of cellular antiviral state. Viral protein synthesis is not affected by the inhibition of the cellular 3' end processing machinery because the poly(A) tails of viral mRNAs are produced by the viral polymerase, through a stuttering mechanism | ||
==External Link== | ==External Link== | ||
pubmed http://www.ncbi.nlm.nih.gov/entrez/utils/fref.fcgi?/pubmed?term=(swine+OR+H1N1)+AND+(flu+OR+influenza+OR+virus+OR+outbreak+OR+pandemic)+AND+%22last+6+months%22[edat] | pubmed http://www.ncbi.nlm.nih.gov/entrez/utils/fref.fcgi?/pubmed?term=(swine+OR+H1N1)+AND+(flu+OR+influenza+OR+virus+OR+outbreak+OR+pandemic)+AND+%22last+6+months%22[edat] | ||
| + | |||
| + | GenBank Sequence http://www.ncbi.nlm.nih.gov/genomes/FLU/SwineFlu.html | ||
| + | All books on influenza http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Search&db=nlmcatalog;term=((influenzavirus+a[mh])+OR+(orthomyxoviridae+infections[mh]))+AND+english[la] | ||
| + | more information http://www.nlm.nih.gov/medlineplus/h1n1fluswineflu.html#cat1 | ||
| + | evoluion of M-gene in influenza virus http://www.virologyj.com/content/6/1/67 | ||
| + | more information http://www.biohealthbase.org/GSearch/home.do?decorator=Influenza | ||
Current revision
Contents |
[edit] Suggestions Invited
[edit] H1N1 Flu (Swine Flu)
Also called: Swine flu
Swine flu is an infection caused by a virus. It's named for a virus that pigs can get. People do not normally get swine flu, but human infections can and do happen. The virus is contagious and can spread from human to human. Symptoms of swine flu in people are similar to the symptoms of regular human flu and include fever, cough, sore throat, body aches, headache, chills and fatigue.
There are antiviral medicines you can take to prevent or treat swine flu. There is no vaccine available right now to protect against swine flu. You can help prevent the spread of germs that cause respiratory illnesses like influenza by
- Covering your nose and mouth with a tissue when you cough or sneeze. Throw the tissue in the trash after you use it.
- Washing your hands often with soap and water, especially after you cough or sneeze. You can also use alcohol-based hand cleaners.
- Avoiding touching your eyes, nose or mouth. Germs spread this way.
- Trying to avoid close contact with sick people.
- Staying home from work or school if you are sick.
[edit] Key Facts
What is Swine Influenza?
Swine Influenza (swine flu) is a respiratory disease of pigs caused by type A influenza virus that regularly causes outbreaks of influenza in pigs. Swine flu viruses cause high levels of illness and low death rates in pigs. Swine influenza viruses may circulate among swine throughout the year, but most outbreaks occur during the late fall and winter months similar to outbreaks in humans. The classical swine flu virus (an influenza type A H1N1 virus) was first isolated from a pig in 1930.
How many swine flu viruses are there?
Like all influenza viruses, swine flu viruses change constantly. Pigs can be infected by avian influenza and human influenza viruses as well as swine influenza viruses. When influenza viruses from different species infect pigs, the viruses can reassort (i.e. swap genes) and new viruses that are a mix of swine, human and/or avian influenza viruses can emerge. Over the years, different variations of swine flu viruses have emerged. At this time, there are four main influenza type A virus subtypes that have been isolated in pigs: H1N1, H1N2, H3N2, and H3N1. However, most of the recently isolated influenza viruses from pigs have been H1N1 viruses.
[edit] Swine Flu in Humans
Can humans catch swine flu?
Swine flu viruses do not normally infect humans. However, sporadic human infections with swine flu have occurred. Most commonly, these cases occur in persons with direct exposure to pigs (e.g. children near pigs at a fair or workers in the swine industry). In addition, there have been documented cases of one person spreading swine flu to others. For example, an outbreak of apparent swine flu infection in pigs in Wisconsin in 1988 resulted in multiple human infections, and, although no community outbreak resulted, there was antibody evidence of virus transmission from the patient to health care workers who had close contact with the patient.
How common is swine flu infection in humans?
In the past, CDC received reports of approximately one human swine influenza virus infection every one to two years in the U.S., but from December 2005 through February 2009, 12 cases of human infection with swine influenza have been reported.
What are the symptoms of swine flu in humans?
The symptoms of swine flu in people are expected to be similar to the symptoms of regular human seasonal influenza and include fever, lethargy, lack of appetite and coughing. Some people with swine flu also have reported runny nose, sore throat, nausea, vomiting and diarrhea.
Can people catch swine flu from eating pork?
No. Swine influenza viruses are not transmitted by food. You can not get swine influenza from eating pork or pork products. Eating properly handled and cooked pork and pork products is safe. Cooking pork to an internal temperature of 160°F kills the swine flu virus as it does other bacteria and viruses.
How does swine flu spread?
Influenza viruses can be directly transmitted from pigs to people and from people to pigs. Human infection with flu viruses from pigs are most likely to occur when people are in close proximity to infected pigs, such as in pig barns and livestock exhibits housing pigs at fairs. Human-to-human transmission of swine flu can also occur. This is thought to occur in the same way as seasonal flu occurs in people, which is mainly person-to-person transmission through coughing or sneezing of people infected with the influenza virus. People may become infected by touching something with flu viruses on it and then touching their mouth or nose.
What do we know about human-to-human spread of swine flu?
In September 1988, a previously healthy 32-year-old pregnant woman was hospitalized for pneumonia and died 8 days later. A swine H1N1 flu virus was detected. Four days before getting sick, the patient visited a county fair swine exhibition where there was widespread influenza-like illness among the swine.
In follow-up studies, 76% of swine exhibitors tested had antibody evidence of swine flu infection but no serious illnesses were detected among this group. Additional studies suggest that one to three health care personnel who had contact with the patient developed mild influenza-like illnesses with antibody evidence of swine flu infection.
How can human infections with swine influenza be diagnosed?
To diagnose swine influenza A infection, a respiratory specimen would generally need to be collected within the first 4 to 5 days of illness (when an infected person is most likely to be shedding virus). However, some persons, especially children, may shed virus for 10 days or longer. Identification as a swine flu influenza A virus requires sending the specimen to CDC for laboratory testing.
What medications are available to treat swine flu infections in humans?
There are four different antiviral drugs that are licensed for use in the US for the treatment of influenza: amantadine, rimantadine, oseltamivir and zanamivir. While most swine influenza viruses have been susceptible to all four drugs, the most recent seven swine influenza viruses isolated from humans are resistant to amantadine and rimantadine. At this time, CDC recommends the use of oseltamivir or zanamivir for the treatment and/or prevention of infection with swine influenza viruses. More information on treatment recommendations can be found at www.cdc.gov/flu/swine/recommendations.htm.
What other examples of swine flu outbreaks are there?
Probably the most well known is an outbreak of swine flu among soldiers in Fort Dix, New Jersey in 1976. The virus caused disease with x-ray evidence of pneumonia in at least 4 soldiers and 1 death; all of these patients had previously been healthy. The virus was transmitted to close contacts in a basic training environment, with limited transmission outside the basic training group. The virus is thought to have circulated for a month and disappeared. The source of the virus, the exact time of its introduction into Fort Dix, and factors limiting its spread and duration are unknown. The Fort Dix outbreak may have been caused by introduction of an animal virus into a stressed human population in close contact in crowded facilities during the winter. The swine influenza A virus collected from a Fort Dix soldier was named A/New Jersey/76 (Hsw1N1).
Is the H1N1 swine flu virus the same as human H1N1 viruses?
No. The H1N1 swine flu viruses are antigenically very different from human H1N1 viruses and, therefore, vaccines for human seasonal flu would not provide protection from H1N1 swine flu viruses.
Swine Flu in Pigs
How does swine flu spread among pigs?
Swine flu viruses are thought to be spread mostly through close contact among pigs and possibly from contaminated objects moving between infected and uninfected pigs. Herds with continuous swine flu infections and herds that are vaccinated against swine flu may have sporadic disease, or may show only mild or no symptoms of infection.
What are signs of swine flu in pigs?
Signs of swine flu in pigs can include sudden onset of fever, depression, coughing (barking), discharge from the nose or eyes, sneezing, breathing difficulties, eye redness or inflammation, and going off feed.
How common is swine flu among pigs?
H1N1 and H3N2 swine flu viruses are endemic among pig populations in the United States and something that the industry deals with routinely. Outbreaks among pigs normally occur in colder weather months (late fall and winter) and sometimes with the introduction of new pigs into susceptible herds. Studies have shown that the swine flu H1N1 is common throughout pig populations worldwide, with 25 percent of animals showing antibody evidence of infection. In the U.S. studies have shown that 30 percent of the pig population has antibody evidence of having had H1N1 infection. More specifically, 51 percent of pigs in the north-central U.S. have been shown to have antibody evidence of infection with swine H1N1. Human infections with swine flu H1N1 viruses are rare. There is currently no way to differentiate antibody produced in response to flu vaccination in pigs from antibody made in response to pig infections with swine H1N1 influenza.
While H1N1 swine viruses have been known to circulate among pig populations since at least 1930, H3N2 influenza viruses did not begin circulating among US pigs until 1998. The H3N2 viruses initially were introduced into the pig population from humans. The current swine flu H3N2 viruses are closely related to human H3N2 viruses.
Is there a vaccine for swine flu?
Vaccines are available to be given to pigs to prevent swine influenza. There is no vaccine to protect humans from swine flu. The seasonal influenza vaccine will likely help provide partial protection against swine H3N2, but not swine H1N1 viruses.
[edit] Antiviral Drugs and H1N1 Flu (Swine Flu)
- Antiviral Drugs
- Benefits of Antiviral Drugs
- CDC Recommendation
[edit] Antiviral Drugs
Photo of female pharmacistAntiviral drugs are prescription medicines (pills, liquid or an inhaler) with activity against influenza viruses, including swine influenza viruses. Antiviral drugs can be used to treat swine flu or to prevent infection with swine flu viruses. These medications must be prescribed by a health care professional. Influenza antiviral drugs only work against influenza viruses -- they will not help treat or prevent symptoms caused by infection from other viruses that can cause symptoms similar to the flu.
There are four influenza antiviral drugs approved for use in the United States (oseltamivir, zanamivir, amantadine and rimantadine). The swine influenza A (H1N1) viruses that have been detected in humans in the United States and Mexico are resistant to amantadine and rimantadine so these drugs will not work against these swine influenza viruses. Laboratory testing on these swine influenza A (H1N1) viruses so far indicate that they are susceptible (sensitive) to oseltamivir and zanamivir.
CDC recommends the use of oseltamivir or zanamivir for the treatment and/or prevention of infection with swine influenza viruses.
- Oseltamivir (brand name Tamiflu ®) is approved to both treat and prevent influenza A and B virus infection in people one year of age and older.
- Zanamivir (brand name Relenza ®) is approved to treat influenza A and B virus infection in people 7 years and older and to prevent influenza A and B virus infection in people 5 years and older.
Recommendations for using antiviral drugs for treatment or prevention of swine influenza will change as we learn more about this new virus.
[edit] Benefits of Antiviral Drugs
Photo of man covering face with tissueTreatment: If you get sick, antiviral drugs can make your illness milder and make you feel better faster. They may also prevent serious influenza complications. Influenza antiviral drugs work best when started soon after illness onset (within two [2] days), but treatment with antiviral drugs should still be considered after 48 hours of symptom onset, particularly for hospitalized patients or people at high risk for influenza-related complications.
[edit] Frequently Asked question about Antiviral Drug
1. For what purposes can antiviral drugs be used against influenza A(H1N1)?
Ans.So far most people who have contracted the new A (H1N1) virus have experienced influenza-like symptoms (such as sore throat, cough, runny nose, fever, malaise, headache, joint/muscle pain) and recovered without antiviral treatment.
Antiviral drugs may reduce the symptoms and duration of illness, just as they do for seasonal influenza. They also may contribute to preventing severe disease and death. Influenza A (H1N1) is a new virus and only a small number of people with the infection have been treated for it with antiviral drugs. WHO is in touch with public health authorities and clinicians in affected countries and is gathering information about how effective the drugs are.
2.To which antiviral drugs does this influenza virus respond?
Ans.There are two classes of antiviral drugs for influenza: inhibitors of neuraminidase such as oseltamivir and zanamivir; and adamantanes, such as amantadine and rimantadine. Tests on viruses obtained from patients in Mexico and the United States have indicated that current new H1N1 viruses are sensitive to neuraminidase inhibitors, but that the viruses are resistant to the other class, the adamantanes.
3.Could the virus become resistant to oseltamivir and zanamivir?
Ans.Resistance can develop to antiviral drugs used for influenza. Therefore, WHO and its partners are monitoring antiviral drug resistance.
4.Under what circumstances should antiviral drugs be administered?
Antiviral drugs are to be used according to national pandemic influenza preparedness plans. Public health authorities in some countries have decided to treat patients likely to have this disease as a part of public health measures.
5.Where antiviral drugs are available for treatment, clinicians should make decisions based on assessment of the individual patient's risk. Risks versus benefits should also be evaluated on a case by case basis.
Should I take an antiviral now just in case I catch the new virus?
Ans.No. You should only take an antiviral, such as oseltamivir or zanamivir, if your health care provider advises you to do so. Individuals should not buy medicines to prevent or fight this new influenza without a prescription, and they should exercise caution in buying antivirals over the internet.
6.What is WHO doing about getting antiviral drugs to countries as preparation for a pandemic?
Ans.WHO’s first priority is to provide an emergency stock of antiviral drugs to countries that have no or insufficient stock of the drugs and lack the capacity to procure these drugs themselves.
WHO is also working with Member States, donors and other groups that have stockpiles and are willing to share these with WHO for distribution to countries in need.
7.Which drug will be provided, and how much of it does WHO have available?
Ans.WHO had a global stockpile of approximately 5 million adult treatment courses of oseltamivir. Part of this stockpile has already been distributed through the WHO Regional Offices, which are handling allocation and distribution. WHO is currently distributing the remaining 3 million adult treatment courses of this stockpile to developing countries in need.
WHO continues to assess needs and to work with manufacturers to secure more donations of antivirals. More antiviral drugs will be distributed once these donations are received.
8.Which countries will receive the drug, and how will they be selected?
Ans.WHO has arranged the first deployment of antiviral drugs from the WHO stockpile to 72 countries. Priority was given to vulnerable countries, taking into consideration national manufacturing and procurement capacity. As necessary, other countries will be supported through regional office stockpiles.
9.What if the initial emergency deployment turns out to be inadequate?
Ans.WHO is in discussion with manufacturers regarding the potential need for scaling up production. It is WHO’s understanding that manufacturers have plans for producing large numbers of treatments quickly.
WHO will work on behalf of its Member States to secure further antivirals as needed, either through donations or purchase at the lowest possible price.
[edit] Vaccine
Swine Flu Vaccine It is estimated that the swine flu vaccine won't be ready until sometime around September to November 2009. In addition to the time required to actually make a new vaccine, the likely need to make seasonal flu vaccine for next year may delay things a little.
Can the swine flu vaccine be combined with the seasonal flu vaccine? No, as vaccine companies will be done making seasonal flu vaccine before they can likely even get started on the swine flu vaccine.
Once the swine flu is ready, who will get it?
CDC’s Advisory Committee on Immunization Practices (ACIP) recommends that swine flu vaccine should first go to:
- Pregnant women
- Household contacts and caregivers for children younger than 6 months of age
- Healthcare and emergency medical services personnel
- All children and young adults from 6 months through 24 years of age, and
- Persons aged 25 through 64 years who have health conditions associated with higher risk of medical complications from influenza
That will take up about 159 million doses of swine flu vaccine. If supplies of swine flu vaccine are limited, priority should go to:
- Pregnant women
- Household contacts and caregivers for children younger than 6 months of age
- Healthcare and emergency medical services personnel
- Children 6 months through 4 years of age, and
- Children 5 through 18 years of age who have chronic medical conditions
Next, as swine flu vaccine improves to the point that all priority groups have gotten vaccinated, everyone from the ages of 25 through 64 years will get vaccinated too. Lastly, people 65 or older, who have the least risk from the swine flu will be offered the swine flu vaccine.
Swine Flu Vaccine Trials
Novartis has started human testing of H1N1 swine flu vaccine candidates while Sanofi-Aventis, the world leader in flu shots, will commence within days, company officials said on Tuesday.
The National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health, will soon start swine flu clinical trials to make sure the new swine flu vaccines are safe and effective. They are to be conducted at eight university research hospitals and medical organizations across the United States, including Baylor College of Medicine in Houston, Children's Hospital Medical Center in Cincinnati, and Emory University in Atlanta.
The first clinical trials will test whether one or two doses are needed and will test both 15mcg and 30mcg doses of vaccine. Although the trials will start in adults, if the vaccines are safe, they will also be tested in children. 1976 Swine Flu Vaccine
Although it is true that we don't currently have a swine flu vaccine, there once was a swine flu vaccine that was made to target the swine flu H1N1 strain that was found at Fort Dix, New Jersey. Because of fears that this swine flu strain was similar to the flu strain that caused the 1918 Spanish Flu pandemic, a vaccination program immunized more than 40 million people in the United States between October 1976 to December 1976.
The immunization program was stopped early because the swine flu pandemic didn't occur, and the swine flu vaccine was thought to cause many side effects, including Guillain-Barre syndrome.
How many cases of Guillain-Barre syndrome were there? About 40, or 1 per million people vaccinated. Even that was considered too high though in light of the fact that there didn't seem to be any cases of swine flu that season.
[edit] Some PDB Structure of H1N1
1RU7 HA Hemagglutinin H1N1 1934 human h1 hemagglutinin AAA43194.1 1RVX HA Hemagglutinin H1N1 1934 h1 hemagglutinin in complex with lsta AAA43194.1 1RVZ HA Hemagglutinin H1N1 1934 h1 hemagglutinin in complex with lstc AAA43194.1 1AA7 M1 Matrix protein 1 H1N1 influenza virus matrix protein crystal structure at ph 4.0 CAA24282.1 1HHI M1 Matrix protein 1 H1N1 the antigenic identity of peptide(slash)mhc complexes: a comparison of the conformation of five peptides presented by hla-a2 AAM75161.1 1EA3 M1 Matrix protein 1 H1N1 influenza virus m1 protein AAM75161.1 2GX9 NS1 Non-structural protein 1 H1N1 x-ray strucutre of influenza virus ns1 effector domain AAA43536.1 1PD3 NS2 Non-structural protein 2 H1N1 influenza a nep m1-binding domain 3BUY PB1-F2 H1N1 mhc-i in complex with peptide ABO21707.1 2HN8 PB1-F2 H1N1 structural characterization and oligomerization of pb1-f2, a pro-apoptotic influenza a virus protein ABO21707.1 2H95 M2 Matrix protein2 H1N1 structure of the amantadine-blocked influenza a m2 proton channel trans-membrane domain by solid-state nmr spectroscopy ABD95352.1 1NYJ M2 Matrix protein2 H1N1 the closed state structure of m2 protein h+ channel by solid state nmr spectroscopy ABF21315.1 2IQH NP Nucleoprotein A/WSN/1933 TS61 H1N1 influenza a virus nucleoprotein np at 3.2a resolution ABF47959.1
Non structural protein NP1 may the the important drug target as concerned from it function. function Inhibits post-transcriptional processing of cellular pre-mRNA, by binding and inhibiting two cellular proteins that are required for the 3'-end processing of cellular pre-mRNAs: the 30 kDa cleavage and polyadenylation specificity factor (CPSF4) and the poly(A)-binding protein 2 (PABPN1). This results in the accumulation of unprocessed 3' end pre-mRNAs which can't be exported from the nucleus. Cellular protein synthesis is thereby shut off very early after virus infection. This protein synthesis shut off is presumably necessary for the virus to escape interferon synthesis after activation of the IRF3 pathway, and therefore prevents establishment of cellular antiviral state. Viral protein synthesis is not affected by the inhibition of the cellular 3' end processing machinery because the poly(A) tails of viral mRNAs are produced by the viral polymerase, through a stuttering mechanism
[edit] External Link
GenBank Sequence http://www.ncbi.nlm.nih.gov/genomes/FLU/SwineFlu.html All books on influenza http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Search&db=nlmcatalog;term=((influenzavirus+a[mh])+OR+(orthomyxoviridae+infections[mh]))+AND+english[la] more information http://www.nlm.nih.gov/medlineplus/h1n1fluswineflu.html#cat1 evoluion of M-gene in influenza virus http://www.virologyj.com/content/6/1/67 more information http://www.biohealthbase.org/GSearch/home.do?decorator=Influenza